Which statement correctly describes the relationship between reactant and yield – Exploring the intricate relationship between reactants and yield, this discussion unveils the fundamental concepts that govern chemical reactions, delving into the factors that influence the efficiency and outcomes of these processes.
Reactants, the initial components of a reaction, undergo transformations to produce yield, the final products. Understanding their interplay is crucial in predicting reaction outcomes and optimizing chemical processes.
1. Definitions and Key Concepts

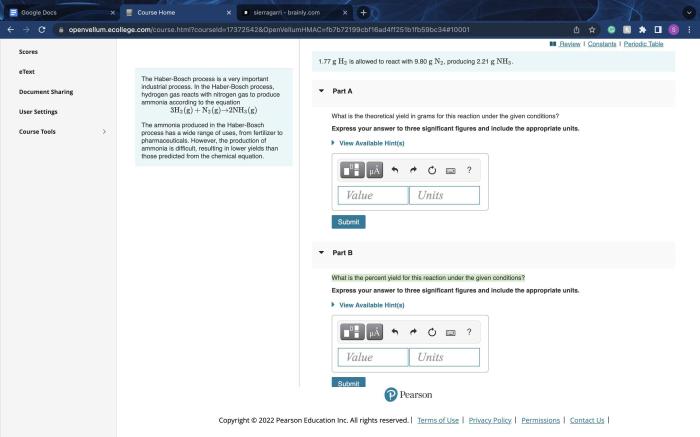
In a chemical reaction, the reactants are the starting materials that undergo a transformation, while the yield refers to the amount of product formed during the reaction. Understanding the relationship between reactants and yield is crucial in chemistry as it allows scientists to predict the outcome of reactions and optimize their efficiency.
For example, in the combustion of methane (CH4), the reactants are methane and oxygen (O2), and the yield is carbon dioxide (CO2) and water (H2O). The balanced chemical equation for this reaction is: CH4 + 2O2 → CO2 + 2H2O.
Stoichiometry, the study of the quantitative relationships between reactants and products in chemical reactions, plays a vital role in determining the yield. Stoichiometric calculations allow chemists to determine the exact amount of reactants needed to produce a desired amount of product.
2. Factors Affecting Yield
The yield of a chemical reaction can be affected by several factors, including:
- Temperature:Higher temperatures generally increase the rate of reaction and, consequently, the yield.
- Concentration:Increasing the concentration of reactants increases the likelihood of collisions between them, leading to a higher yield.
- Surface area:For reactions involving solids, increasing the surface area of the solid reactant increases the number of active sites available for reaction, resulting in a higher yield.
- Catalysts:Catalysts are substances that increase the rate of a reaction without being consumed themselves. They provide an alternative pathway for the reaction to occur, lowering the activation energy and increasing the yield.
- Limiting reactants:In reactions involving multiple reactants, the reactant present in the smallest molar ratio relative to its stoichiometric requirement is the limiting reactant. The limiting reactant determines the maximum yield that can be obtained from the reaction.
3. Reaction Pathways and Mechanisms

Chemical reactions can proceed through different pathways and mechanisms. A reaction pathway refers to the sequence of steps that lead from the reactants to the products, while the reaction mechanism describes the detailed molecular interactions that occur during each step.
For example, the combustion of methane can occur through two different pathways:
- Single-step pathway:CH4 + 2O2 → CO2 + 2H2O
- Two-step pathway:CH4 + O2 → CH3OH + H2O; CH3OH + O2 → CO2 + 2H2O
The reaction mechanism for each pathway involves a series of elementary reactions, which are individual steps that involve the breaking and forming of chemical bonds.
The reaction pathway and mechanism can influence the yield of the desired product. For example, in the combustion of methane, the two-step pathway may result in a lower yield of CO2 if the intermediate product, methanol (CH3OH), undergoes further reactions before it can be converted to CO2.
4. Optimization of Yield

Optimizing the yield of a chemical reaction is crucial in various fields, such as chemical engineering, pharmaceutical manufacturing, and environmental chemistry. Several strategies can be employed to enhance yield:
- Using catalysts:Catalysts can significantly increase the rate of reaction and, consequently, the yield.
- Controlling temperature:Maintaining the optimal temperature for the reaction can maximize the yield.
- Adjusting reactant concentrations:Ensuring that the reactants are present in the correct stoichiometric ratios can optimize yield.
- Increasing surface area:For reactions involving solids, increasing the surface area of the solid reactant can enhance yield.
- Removing inhibitors:Inhibitors are substances that slow down or prevent reactions. Identifying and removing inhibitors can improve yield.
Successful yield optimization has been demonstrated in various real-world applications. For example, in the production of sulfuric acid, the use of vanadium pentoxide (V2O5) as a catalyst has significantly increased the yield and efficiency of the process.
5. Applications and Implications
Understanding the relationship between reactants and yield has practical applications in numerous fields:
- Chemical engineering:Optimizing chemical reactions to maximize product yield is essential in industrial processes.
- Pharmaceutical manufacturing:Controlling the yield of pharmaceutical reactions is crucial for ensuring the production of safe and effective drugs.
- Environmental chemistry:Understanding the yield of reactions involving pollutants can help develop strategies to minimize their environmental impact.
Furthermore, yield optimization has implications for sustainability and resource management. By maximizing the yield of reactions, industries can reduce waste, conserve resources, and promote sustainable practices.
Answers to Common Questions: Which Statement Correctly Describes The Relationship Between Reactant And Yield
What is the significance of stoichiometry in determining yield?
Stoichiometry provides the quantitative relationships between reactants and products in a chemical reaction, allowing for precise prediction of yield based on the initial amounts of reactants.
How does temperature affect yield?
Temperature can influence reaction rates and equilibrium positions, thereby affecting yield. Higher temperatures generally favor reactions with higher activation energies, while lower temperatures promote reactions with lower activation energies.
What is the role of limiting reactants in yield optimization?
Limiting reactants are those that are consumed completely in a reaction, limiting the amount of product that can be formed. Identifying and manipulating limiting reactants is crucial for maximizing yield.