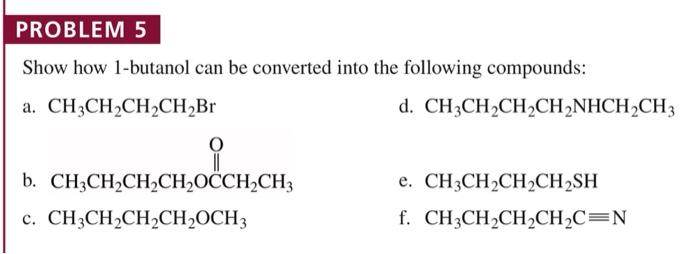
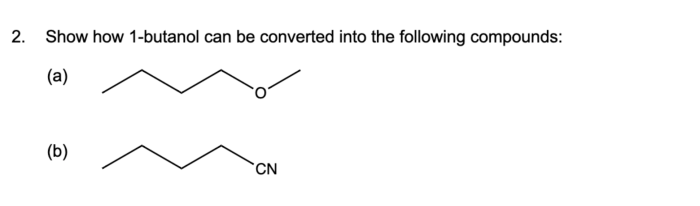
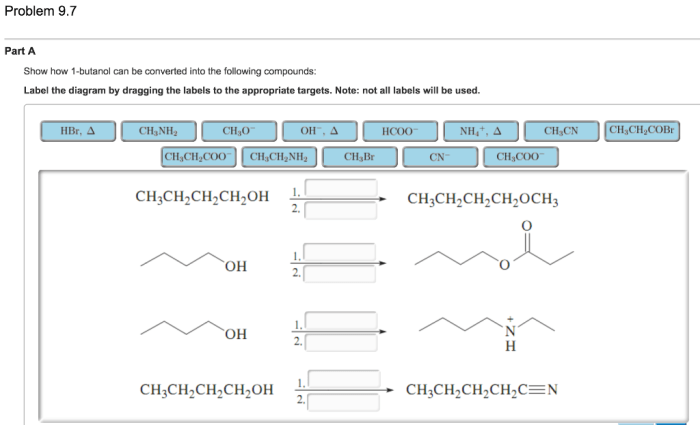
Show how 1-butanol can be converted into the following compounds, embarking on a scientific expedition that unravels the fascinating chemistry behind these transformations. From oxidation to dehydration, substitution to elimination, this journey explores the diverse reactions that unlock the potential of 1-butanol.
Delving into the intricacies of each reaction, we uncover the mechanisms, catalysts, and conditions that govern these conversions. Witness the versatility of 1-butanol as it undergoes oxidation to yield butanal, dehydration to form 1-butene, nucleophilic substitution to produce sodium butanoate, and elimination to generate 2-butene.
1-Butanol Conversion
-butanol, a four-carbon alcohol, serves as a versatile starting material for various organic transformations. This article delves into the key reactions that convert 1-butanol into different compounds, providing detailed mechanisms and discussing reaction conditions.
Oxidation of 1-Butanol

Oxidizing 1-butanol yields butanal, an aldehyde. This reaction involves the addition of an oxygen atom to the carbon adjacent to the hydroxyl group. Typically, strong oxidizing agents like potassium permanganate (KMnO4) or chromic acid (H2CrO4) are employed in acidic conditions.
The reaction proceeds via an intermediate hemiacetal, which subsequently dehydrates to form butanal.Chemical Equation:CH3(CH2)3OH + [O] → CH3(CH2)3CHO + H2O
Dehydration of 1-Butanol: Show How 1-butanol Can Be Converted Into The Following Compounds
Dehydration of 1-butanol leads to the formation of 1-butene, an alkene. This reaction involves the removal of a water molecule from the alcohol. Acid catalysts, such as sulfuric acid (H2SO4) or phosphoric acid (H3PO4), facilitate protonation of the hydroxyl group, creating a carbocation intermediate.
This carbocation then undergoes elimination of a proton to form 1-butene.Reaction Mechanism:
- CH3(CH2)3OH + H+ → CH3(CH2)3OH2+
- CH3(CH2)3OH2+ → CH3(CH2)2CH=CH2 + H2O
Substitution Reactions of 1-Butanol
-butanol undergoes nucleophilic substitution reactions, where the hydroxyl group is replaced by another nucleophile. A classic example is the reaction with sodium hydroxide (NaOH), which yields sodium butanoate, a salt. This reaction occurs in aqueous or alcoholic solutions, and the rate is influenced by the concentration of the nucleophile and the temperature.Chemical
Equation:CH3(CH2)3OH + NaOH → CH3(CH2)3COONa + H2O
Elimination Reactions of 1-Butanol
Elimination reactions of 1-butanol result in the formation of alkenes. One such reaction is the elimination of 2-butene using potassium tert-butoxide (t-BuOK) as a base. This reaction proceeds via an E2 mechanism, where the base abstracts a proton from the carbon adjacent to the hydroxyl group, leading to the formation of a carbanion intermediate.
The carbanion then eliminates a proton from the adjacent carbon to form 2-butene.Chemical Equation:CH3(CH2)3OH + t-BuOK → CH3CH=CHCH3 + t-BuOH
Frequently Asked Questions
What is the significance of converting 1-butanol into different compounds?
These conversions provide access to a range of valuable chemicals used in various industries, including pharmaceuticals, solvents, and fragrances.
How does the reaction mechanism influence the choice of reaction conditions?
Understanding the reaction mechanism helps optimize reaction conditions, such as temperature, pressure, and catalyst selection, to achieve desired yields and selectivities.