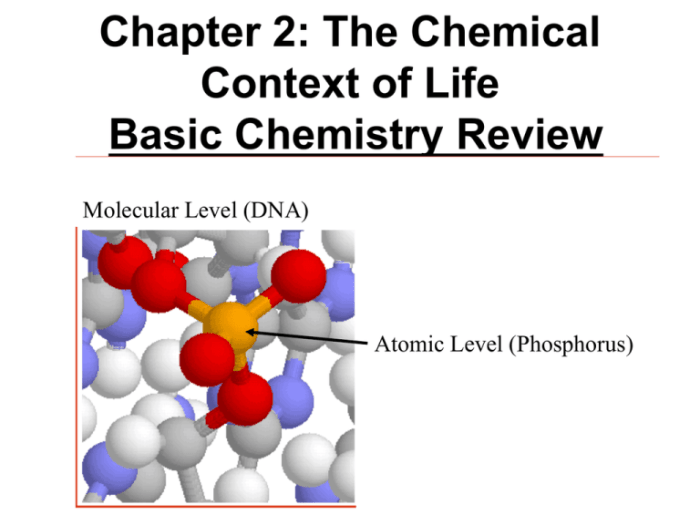
Chapter 2 active reading guide the chemical context of life – Embark on an enthralling journey with Chapter 2 Active Reading Guide: The Chemical Context of Life, a comprehensive exploration of the fundamental principles that govern the intricate workings of living organisms.
This guide unveils the building blocks of life, delving into the chemical composition of living organisms and the structure and function of biomolecules. It illuminates the vital role of chemical reactions and energy flow in biological systems, providing a deeper understanding of the dynamic processes that sustain life.
Chapter Overview

Chapter 2 provides an in-depth examination of the chemical context of life. It emphasizes the importance of understanding the fundamental chemical principles that govern the structure and function of living organisms. By exploring the chemical composition, biomolecular structures, and chemical reactions that occur within living systems, this chapter lays the groundwork for comprehending the complex processes of life.
Chemical Composition of Living Organisms
Living organisms are composed of a diverse array of elements, with the majority consisting of carbon, hydrogen, oxygen, nitrogen, and phosphorus (CHNOP). These elements form various compounds, including water, carbohydrates, proteins, lipids, and nucleic acids, which are essential for life’s functions.
| Element | Symbol | Function |
|---|---|---|
| Carbon | C | Backbone of organic molecules |
| Hydrogen | H | Forms covalent bonds with carbon |
| Oxygen | O | Essential for respiration and metabolism |
| Nitrogen | N | Found in proteins and nucleic acids |
| Phosphorus | P | Component of nucleic acids and energy molecules |
Structure and Function of Biomolecules
- Carbohydrates:Energy sources and structural components (e.g., glucose, cellulose)
- Proteins:Catalyze reactions, provide structural support, and transport molecules (e.g., enzymes, collagen, hemoglobin)
- Lipids:Energy storage, insulation, and membrane formation (e.g., fats, oils, phospholipids)
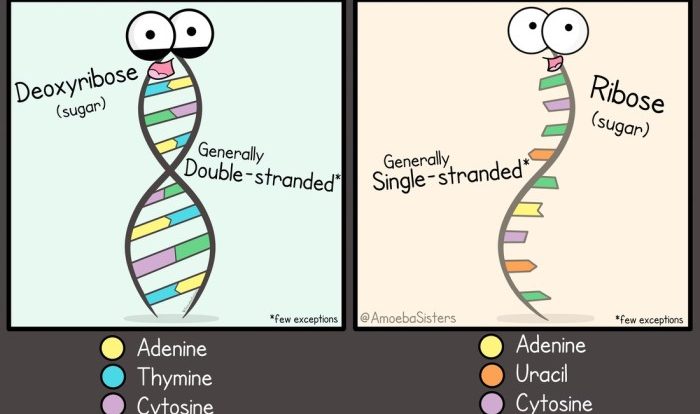
- Nucleic acids:Store and transmit genetic information (e.g., DNA, RNA)
Chemical Reactions in Living Organisms, Chapter 2 active reading guide the chemical context of life
Chemical reactions are essential for life processes, including metabolism, energy production, and growth. Enzymes facilitate these reactions by lowering the activation energy required for reactions to occur.
- Catabolism:Breaks down complex molecules to release energy
- Anabolism:Builds complex molecules from simpler ones
- Metabolism:Sum of all chemical reactions in an organism
Energy Flow in Biological Systems
Free energy is the energy available to do work. Biological systems utilize energy through photosynthesis and cellular respiration, converting light energy or chemical energy into ATP, the universal energy currency of cells.
Homeostasis and the Chemical Context of Life
Homeostasis refers to the maintenance of a stable internal environment despite external changes. Chemical reactions play a crucial role in maintaining homeostasis, regulating pH, temperature, and other vital parameters.
Expert Answers: Chapter 2 Active Reading Guide The Chemical Context Of Life
What is the significance of understanding the chemical context of life?
Understanding the chemical context of life provides a foundation for comprehending the fundamental processes that govern living organisms. It enables us to unravel the mechanisms behind biological functions, diseases, and potential therapeutic interventions.
How do biomolecules contribute to the structure and function of cells?
Biomolecules, such as carbohydrates, proteins, lipids, and nucleic acids, play crucial roles in determining the structure and function of cells. They form essential components of cell membranes, enzymes, genetic material, and energy storage molecules, enabling cells to carry out their diverse functions.
What is the role of chemical reactions in maintaining homeostasis?
Chemical reactions are essential for maintaining homeostasis, the stable internal environment necessary for life. These reactions regulate body temperature, pH levels, and electrolyte balance, ensuring that cells can function optimally within a narrow range of conditions.