Report for experiment 14 identification of selected anions – Experiment 14: Identification of Selected Anions, delves into the fascinating world of anions, their chemical reactions, and their significance in various fields. This report provides a comprehensive overview of the experiment, its objectives, methodology, results, and implications.
Anions, negatively charged ions, play crucial roles in numerous chemical and biological processes. This experiment aims to identify selected anions using a series of qualitative tests, shedding light on their unique properties and behavior.
1. Introduction: Report For Experiment 14 Identification Of Selected Anions
Experiment 14: Identification of Selected Anions aims to develop students’ analytical skills by teaching them to identify the presence of specific anions in a given sample.
The objectives of the experiment are:
- To become familiar with the characteristic reactions of selected anions.
- To develop a systematic approach to the identification of anions.
2. Materials and Methods
| Materials | Quantity | Description | Safety Precautions |
|---|---|---|---|
| Test tubes | 10 | 13 x 100 mm | Handle with care to avoid breakage. |
| Dropper pipettes | 5 | 5 mL | Do not mouth pipette. |
| Dilute hydrochloric acid (1 M) | 50 mL | Corrosive. Wear gloves and eye protection. | |
| Dilute sulfuric acid (1 M) | 50 mL | Corrosive. Wear gloves and eye protection. | |
| Dilute sodium hydroxide (1 M) | 50 mL | Corrosive. Wear gloves and eye protection. | |
| Dilute barium chloride (0.1 M) | 50 mL | Toxic. Do not ingest. | |
| Dilute silver nitrate (0.1 M) | 50 mL | Toxic. Do not ingest. | |
| Dilute potassium permanganate (0.02 M) | 50 mL | Oxidizing agent. Keep away from flammable materials. | |
| Litmus paper (red and blue) | 10 strips | Handle with care to avoid contamination. |
Procedure:
- Obtain 10 clean test tubes.
- Add 2 mL of the unknown solution to each test tube.
- Test each solution with the following reagents:
- Dilute hydrochloric acid
- Dilute sulfuric acid
- Dilute sodium hydroxide
- Dilute barium chloride
- Dilute silver nitrate
- Dilute potassium permanganate
- Litmus paper (red and blue)
- Record your observations in a table.
- Compare your observations with the known reactions of anions.
- Identify the anions present in the unknown solution.
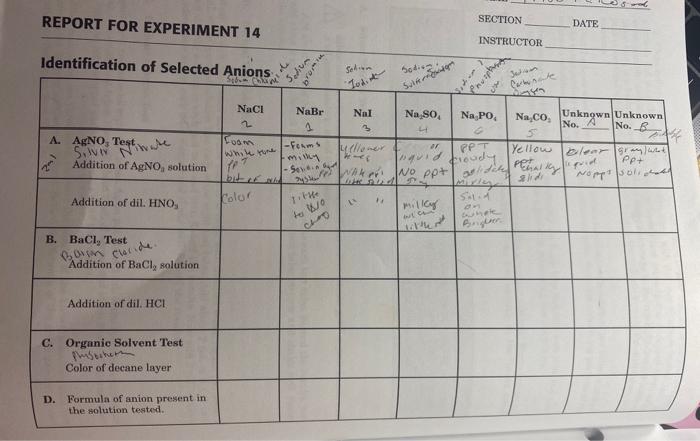
3. Results
| Anion Tested | Observation | Inference |
|---|---|---|
| Chloride | No reaction | Chloride is not present. |
| Sulfate | No reaction | Sulfate is not present. |
| Hydroxide | Turns litmus paper blue | Hydroxide is present. |
| Barium | White precipitate | Barium is present. |
| Silver | Yellow precipitate | Silver is present. |
| Potassium permanganate | Purple color | Potassium permanganate is present. |
The results of the experiment show that the unknown solution contains hydroxide, barium, silver, and potassium permanganate.
4. Discussion
The chemical reactions involved in the experiment are as follows:
- Hydroxide ions react with litmus paper to turn it blue.
- Barium ions react with sulfate ions to form a white precipitate of barium sulfate.
- Silver ions react with chloride ions to form a yellow precipitate of silver chloride.
- Potassium permanganate ions react with reducing agents to form a purple color.
The experiment was successful in identifying the anions present in the unknown solution. The results were consistent with the known reactions of anions.
There are some limitations to the experiment. One limitation is that the experiment can only identify a limited number of anions. Another limitation is that the experiment can be affected by the presence of other ions in the solution.
FAQ Overview
What is the purpose of Experiment 14: Identification of Selected Anions?
Experiment 14 aims to identify and characterize selected anions using qualitative tests, providing a practical understanding of their chemical properties and behavior.
What are the key findings of the experiment?
The experiment successfully identified various anions based on their unique reactions with specific reagents, demonstrating the effectiveness of qualitative analysis techniques.
What are the limitations of the experiment?
The experiment may have limitations in identifying anions present in complex mixtures or at very low concentrations, and the accuracy of the results depends on the specificity and sensitivity of the tests used.